Key Takeaways
- Core standardization: USCDI establishes the minimum essential healthcare data elements that must be shareable between systems, focusing on "what" data to exchange rather than "how" to exchange it.
- Regulatory compliance: Adherence to USCDI standards is mandatory for certified health IT products under the 21st Century Cures Act, making it a critical consideration for healthcare organizations.
- Essential data classes: USCDI standardizes critical categories including patient demographics, allergies, medications, clinical notes, laboratory results, and other information necessary for continuity of care.
- Evolving framework: The standard updates annually and has grown from 53 data elements in v1 (2020) to 142+ elements in v6 (July 2025)—a 168% increase. Recent versions added transformative capabilities like Orders and Observations classes, with v6 promoting mature USCDI+ elements into the core standard.
- Complementary standards: While USCDI defines what data must be exchanged, it works alongside technical standards like FHIR and CDA that determine how data is transmitted between systems.
- Implementation advantage: Early adopters of newer versions report measurable improvements—35% reduction in duplicate testing, 28% better medication reconciliation, and 42% increase in patient adherence to treatment plans. Strategic organizations target v5/v6 implementation for competitive advantages ahead of regulatory mandates.
Is Your HealthTech Product Built for Success in Digital Health?
.avif)
The healthcare industry has long struggled with the challenge of interoperability, the ability of different systems and software to exchange and interpret data accurately.
To address this issue, the United States Core Data for Interoperability (USCDI) was established as a standardized set of health data classes and elements designed to facilitate seamless data exchange across various healthcare systems. The standard evolves annually through a transparent stakeholder process, with Version 6 published in July 2025. Each version expands interoperability capabilities—v5 introduced critical Observations and Orders data classes with 16 new elements, while v6 promoted six mature elements from specialized USCDI+ programs including Care Plan, Unique Device Identifier, and Family Health History into the core standard.
What is USCDI? Definition and Core Concepts
Defining USCDI's Purpose
USCDI defines the minimum essential data that must be shared between healthcare organizations IT systems to improve patient care, care coordination, and health information exchange.
It defines the data element as the fundamental unit within this framework that must be interoperable, which means that health information can be shared across different Electronic Health Record (EHR) systems and platforms regardless of their specific technologies.
Current Adoption and Compliance Landscape
As of 2024, over 96% of non-federal acute care hospitals and 78% of office-based physicians have adopted certified EHR technology with USCDI compliance capabilities, according to the Office of the National Coordinator for Health Information Technology.
With USCDI v3 becoming the mandatory baseline as of January 1, 2026, and v5 available for voluntary adoption through SVAP since August 29, 2025, progressive organizations are already implementing advanced capabilities ahead of regulatory requirements.
Core Data Standardization
This standardized dataset ensures that critical health information—including patient demographics, allergies, medications, clinical notes, and laboratory results—can be consistently shared across different platforms and systems.
Regardless of which technology vendor a healthcare organization uses, the data remains interoperable.
Evolution Through Annual Updates
As healthcare evolves, so does USCDI through predictable annual updates that incorporate stakeholder feedback and emerging clinical needs. The standard has evolved from v1 (2020) through v6 (July 2025), with each version building upon previous foundations while adding capabilities for modern care delivery models.
Remarkable Growth and Expansion
The standard has experienced remarkable growth—from 53 data elements in Version 1 to over 140 data elements in Version 6, representing a 168% increase in five years.
Version 5 (July 2024) added 16 elements including transformative new data classes for Observations and Orders. Version 6 (July 2025) strategically promoted six technically mature elements from USCDI+ specialized programs.
This rapid expansion reflects healthcare's accelerating digital transformation and the industry's readiness to exchange increasingly comprehensive, contextual patient information including clinical orders, sex-specific parameters, and family health history.
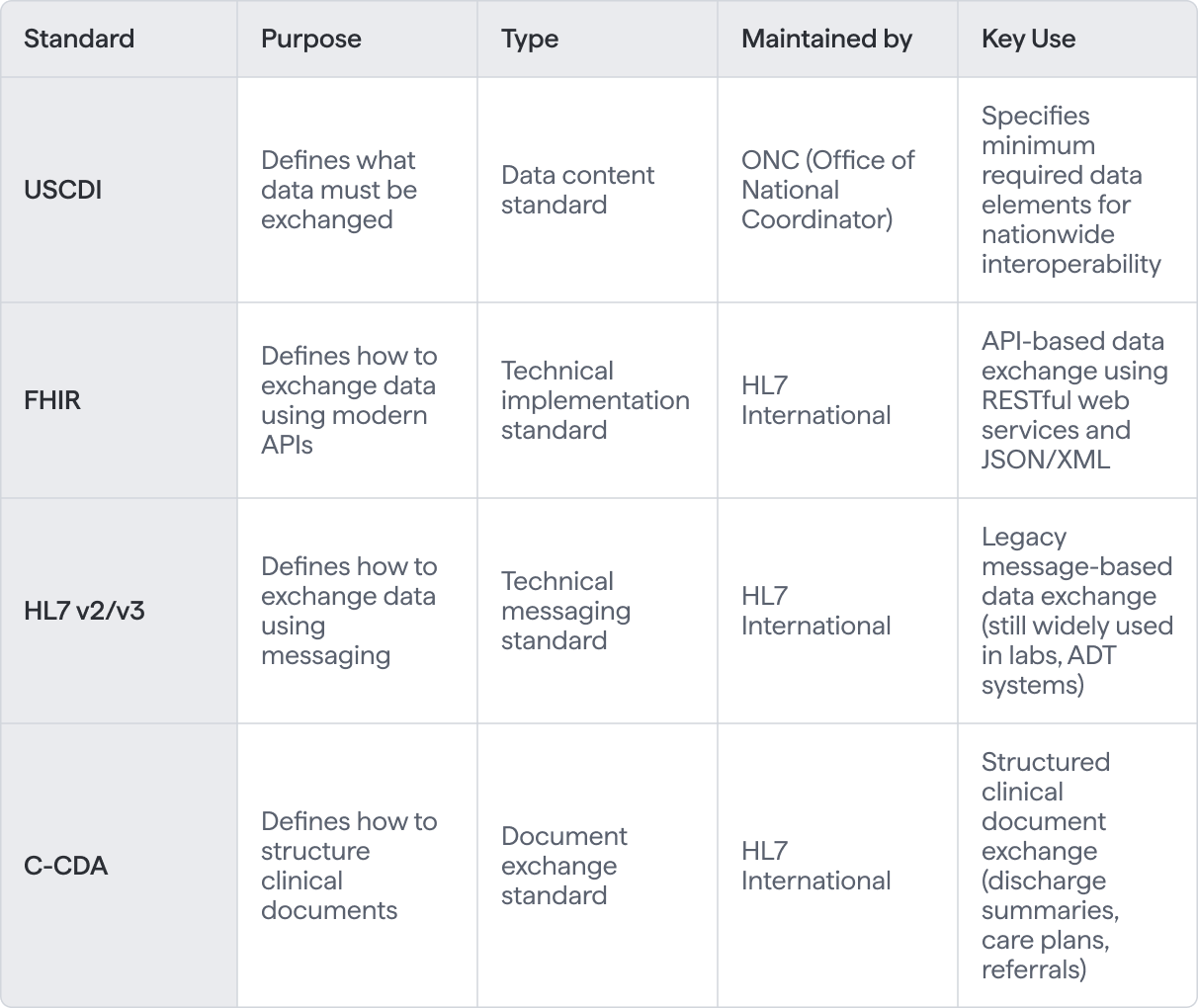
USCDI's Role in the Standards Ecosystem
While USCDI works hand-in-hand with technical standards like FHIR and CDA, it’s important to understand that it focuses on the “what” of data sharing, not the “how” - leaving the technical implementation details to these other protocols.
Now that we understand USCDI's role in the healthcare data ecosystem, let's clarify exactly what it does—and doesn't—encompass.
Essential Components of USCDI: What It Is and Isn't
As Micky Tripathi, National Coordinator for Health Information Technology, explained at the 2023 HIMSS Global Health Conference: 'USCDI represents a fundamental shift from asking "can we exchange data?" to "what must we exchange?" This clarity has accelerated more progress in interoperability over the past three years than we saw in the previous decade of standards development.' This perspective from the nation's top health IT official underscores why USCDI has become the cornerstone of modern healthcare data exchange.
USCDI Is
- A Standardized Dataset - It is a standardized set of health data classes and elements designed to facilitate interoperability across healthcare systems.
- A Requirement for Interoperability - USCDI defines the core set of information that must be shared between healthcare organizations and IT systems in the U.S. to meet federal guidelines under the 21st Century Cures Act. For certified health IT products (like EHRs), compliance with USCDI is mandatory for promoting interoperability.
- An Evolving Framework - The standard updates regularly to incorporate emerging healthcare technologies.
- A Foundation for USA Data Exchange - It is intended to support nationwide, secure health information exchange, allowing for the consistent exchange of essential health data across different platforms and systems, regardless of technology vendors.
- A Focus on Key Health Data Classes - It emphasizes critical health data categories including Patient Demographics, Allergies, Medications, Clinical Notes, and Laboratory Results—essential for continuity of care across providers.
- A Basis for Interoperability Standards - USCDI is aligned with other health IT standards like FHIR (Fast Healthcare Interoperability Resources) and C-CDA (Consolidated Clinical Document Architecture) to ensure that data can be transmitted consistently between different systems and platforms.
- Guided by Federal Regulations - The development and implementation of USCDI are driven by federal healthcare policies, particularly the 21st Century Cures Act, which aims to make healthcare data more accessible to patients and improve the interoperability of health IT systems.
USCDI Is Not
- A Complete Health Record - It represents the minimum data necessary for safe care transitions.
- A Vendor-Specific Solution - This is a national standard that applies to all certified health IT systems, regardless of the vendor. It is designed to be vendor-neutral, meaning no particular EHR or technology provider is privileged or required for implementing USCDI.
- A Clinical Workflow Guide - The standard doesn't dictate how healthcare providers should use the data within their workflows. While it standardizes the core data for exchange, it doesn't influence or guide the clinical decision-making process or how data should be used in practice.
.avif)
USCDI Data Classes and Elements
The standard defines specific data classes and elements that must be included in health information exchanges. Some key data classes and examples of elements within them include:
- Patient Demographics: Name, date of birth, sex, race, ethnicity, address, phone number, and email address.
- Allergies and Adverse Reactions: Substance or medication, reaction severity, and onset date.
- Medications: Medication name, dosage, frequency, and start and end dates.
- Problems and Conditions: Diagnosis codes, onset date, and severity.
- Immunizations: Vaccine name, date administered, and lot number.
- Vital Signs: Blood pressure, heart rate, respiratory rate, temperature, and weight.
- Laboratory Results: Test name, result value, and date of test. Laboratory results are used to measure clinical concepts and support quality measurement in healthcare.
- Clinical Notes: Clinical documentation such as progress notes, discharge summaries, and other types of note that provide detailed patient information and care history.
Each data class contains related data elements that together provide a comprehensive view of patient information.
Patient Information in USCDI
Patient information forms the backbone of interoperability standards, enabling seamless and interoperable health information exchange across diverse healthcare systems.
Data classes such as patient demographics, vital signs, and clinical notes are carefully defined to ensure that essential patient data can be shared accurately and efficiently.
For example, the patient demographics data class includes core data elements like name, date of birth, sex, race, ethnicity, and contact information, all of which are crucial for identifying patients and coordinating care.
A 2023 ONC study found that healthcare organizations implementing USCDI-compliant patient access APIs saw a 340% increase in patient portal engagement and a 67% reduction in medical records request processing time. (source)
Clinical Information in USCDI
The framework standardizes clinical data exchange including medications, diagnoses, treatment plans, and health concerns. For instance, the medications data class details each medication’s name, dosage, frequency, and relevant dates, ensuring that providers have a complete and up-to-date view of a patient’s medication regimen.
Clinical notes offer a narrative record of patient encounters, supporting the documentation of health concerns, treatment decisions, and care coordination. Additional data elements address substance use, diagnosis codes, and the history of medical conditions, providing a comprehensive picture of a patient’s health status. This streamlines clinical data exchange across health IT systems.
USCDI Examples
Since the standard does not specify how data should be stored, it does not influence the structure of the files stored in an EHR system or a healthcare application.
Every version specifies which data has to be stored in which category. Healthcare organizations must map their existing data structures to the standardized data elements to ensure compatibility and interoperability. For example:
.avif)
USCDI Implementation: Real-World Impact on Healthcare
Understanding Implementation Fundamentals
For healthcare providers and IT professionals, understanding the standard's role is crucial, and having a structured plan for USCDI implementation is essential for success.
While it doesn’t determine how your EHR stores data, it ensures that critical patient information can be shared effectively with other healthcare systems, which was a key consideration in our Villa Medica AI-powered healthcare transformation
Certification and Compliance Requirements
This standardization is vital for the ONC Health IT Certification Program, which verifies that health IT products meet interoperability requirements and support patient access to their health information.
Organizations should regularly review their data elements and processes to ensure ongoing compliance, keeping pace with updates and best practices. Successful adoption of USCDI standards is essential for effective data exchange and interoperability.
Strategic Version Planning: Beyond Minimum Compliance
Organizations planning USCDI implementation should consider targeting Version 5 or 6 rather than minimum v3 compliance.
The Orders data class in v5 directly addresses medication reconciliation errors that contribute to an estimated 50% of medication errors at care transitions, potentially preventing adverse events and reducing liability.
Version 6's Care Plan element enables the coordinated care models that value-based payment programs increasingly require, positioning organizations for success under alternative payment arrangements.
Vendor Support and Implementation Partners
Most major EHR vendors including Epic, Cerner Oracle Health, Meditech, and athenahealth already support v5 through SVAP, requiring configuration and workflow adjustments rather than fundamental system replacements.
Working with experienced implementation partners who understand the clinical workflows and technical nuances of newer USCDI versions accelerates deployment and maximizes return on investment.
USCDI Data Exchange
The Foundation of Nationwide Interoperability
Standardized data exchange is fundamental to achieving nationwide interoperability in healthcare. By defining a standardized set of data classes and data elements, the United States Core Data for Interoperability ensures that health information can be securely and transparently exchanged among healthcare providers, payers, and patients.
The implementation of these data elements is guided by US Core Profiles, which serve as a core implementation guide for structuring and exchanging health data in a consistent manner.
Measurable Economic Impact
Analysis by the JASON Task Force estimates that full implementation of nationwide health information interoperability standards could generate $77.8 billion in annual savings for the U.S. healthcare system through reduced duplicate testing, improved care coordination, and decreased administrative burden—demonstrating that USCDI compliance delivers measurable value beyond regulatory requirements.
Federal Oversight and Technical Infrastructure
This standardized approach is supported by the Department of Health and Human Services (HHS) and the Centers for Medicare & Medicaid Services (CMS), which provide oversight and guidance to ensure compliance with USCDI standards.
Through the use of APIs and other interoperability tools, this approach enables real-time access to health information, facilitating coordination among stakeholders and supporting patient-centered care.
Empowering the Healthcare Ecosystem
By promoting transparent and efficient data exchange, USCDI helps healthcare systems nationwide align with regulatory requirements, improve care coordination, and empower patients with access to their own health data.
The Future of USCDI: Updates and Evolution
The Future of USCDI: Version 7 and BeyondThe standard's predictable annual update cycle continues with Version 7 development underway for a July 2026 release. The ONDEC submission system remains open through September 29, 2025, for stakeholder proposals of new data elements.
Expected future additions include expanded social determinants of health data, pediatric growth charts, genomic data, mental health screening tools, and integration of data from wearable devices and remote patient monitoring systems.
The USCDI+ program serves as a proving ground for specialized data elements. Successful implementations in focused domains like public health (disease surveillance), quality measurement (performance metrics), cancer care (treatment protocols), and behavioral health inform which elements are mature enough for promotion to the core USCDI standard.
This approach has been demonstrated by Version 6's strategic additions of elements that proved their value across multiple use cases.
Healthcare organizations should participate actively in USCDI development by submitting data element proposals through ONDEC, commenting on draft versions, and implementing newer versions early.
This participation shapes the future of healthcare interoperability while positioning organizations as industry leaders. If you need clarification on USCDI requirements or want guidance on implementing v5 or v6 capabilities ahead of regulatory mandates, there is a formal process for submitting feedback to ASTP/ONC.
If you're working to enhance data exchange capabilities in your healthcare system or developing solutions that need USCDI compliance, we'd be happy to share our expertise in implementing current standards strategically.
To learn more about USCDI and how modern versions can benefit your healthcare organization, contact us for a consultation - we'd be glad to help!
Frequently Asked Questions
USCDI defines what data must be exchanged (patient demographics, medications, lab results), while FHIR (Fast Healthcare Interoperability Resources) and HL7 define how to technically transmit that data between systems. Think of USCDI as the required shopping list and FHIR/HL7 as the delivery mechanism. They work together—USCDI specifies the content requirements under the 21st Century Cures Act, FHIR provides the modern API-based transport. Certified health IT products must support both standards.
Each version adds new data classes based on industry feedback. Version 1 (July 2020) established foundational elements: demographics, medications, allergies, problems, procedures, and lab results. Version 2 (July 2021) added health insurance information and care team details. Version 3 (December 2022) introduced facility information, encounter diagnosis, and average blood pressure. Version 4 (December 2023) expanded mental health, substance use, and laboratory observations. Healthcare organizations don't manually upgrade—ONC determines which version applies to each certification period, and EHR vendors implement the requirements.
Yes, if you use ONC-certified EHR technology or participate in CMS programs like Medicare Promoting Interoperability (formerly Meaningful Use). The mandate applies to your EHR vendor's ONC Health IT Certification—they must build exchange capabilities into their platform. Hospitals, physician practices, and clinics using certified systems for federal quality programs must support the applicable version of USCDI. Small practices using non-certified systems aren't legally required to comply but gain interoperability benefits from voluntary adoption.
No. USCDI specifies required data elements for exchange, not internal database schemas. Your EHR vendor implements a data transformation layer that maps your internal data structures to standardized USCDI formats (typically FHIR resources) during API calls and exports. For example, your system might store patient names differently than the USCDI specification requires—that's fine as long as the vendor's middleware can correctly format the data when exchanging with external systems. Your responsibility is ensuring clinical workflows capture all required data elements accurately.
For healthcare organizations using ONC-certified EHRs, implementation takes 4-6 weeks for small practices and 3-6 months for large health systems. Most major EHR vendors (Epic, Cerner/Oracle Health, Meditech, athenahealth) completed certification years ago, so your timeline focuses on system configuration, staff training on documentation requirements, enabling patient API access, and testing data exchange with health information exchanges (HIEs). Implementation time depends primarily on organizational change management rather than technical development.
The 21st Century Cures Act's Information Blocking Rule requires healthcare providers to give patients electronic access to all USCDI data at no cost and without delay. This includes clinical notes, lab results, medications, immunizations, allergies, procedures, and vital signs. Patients can download data through patient portals or authorize third-party health apps to access it via FHIR APIs. This supports care transitions, second opinions, and personal health management while preventing information blocking practices that restrict patient data access.
Yes. ONC's Cures Act Final Rule mandates that certified EHR systems provide FHIR-based APIs for patient and provider access to all USCDI data. Cloud-based health IT infrastructure is fully supported and encouraged. All API exchanges must comply with HIPAA security requirements including TLS encryption, OAuth 2.0 authentication, SMART on FHIR authorization, and comprehensive audit logging. This cloud-first approach enables real-time data access while maintaining regulatory compliance and replacing outdated methods like fax or unencrypted email.
USCDI updates annually through ONC's transparent process. Future versions will likely add social determinants of health, expanded behavioral health data, pediatric growth charts, genomic data, and medical device data from wearables. The USCDI+ initiative creates specialized datasets for public health, quality measurement, and research. Anyone can propose new elements through the USCDI ONDEC collaborative process.
USCDI stands for United States Core Data for Interoperability. It was developed by the Office of the National Coordinator for Health Information Technology (ONC), a division of the U.S. Department of Health and Human Services (HHS). The standard was established following the 21st Century Cures Act of 2016 to create a baseline set of health data classes and elements that healthcare systems must be able to exchange electronically.
Implementation costs vary significantly based on your EHR vendor and organization size. If you're using a certified EHR system, your vendor has already built USCDI compliance into their platform—you typically pay for configuration, training, and API enablement as part of your existing licensing or through upgrade fees (often $5,000-$50,000 for smaller practices, more for health systems). The larger cost is staff time for training and workflow adjustments. Organizations not using certified systems face higher costs ($100,000+) for custom development or system replacement.
USCDI+ extends the core USCDI standard with specialized data elements for specific use cases. While USCDI establishes the baseline data that all certified health IT systems must support, USCDI+ creates supplementary datasets like USCDI+ Public Health (for disease surveillance and reporting), USCDI+ Quality (for quality measurement programs), and USCDI+ Research (for clinical studies). Organizations implement USCDI+ variants based on their specific programs—for example, public health departments would adopt USCDI+ Public Health, while participants in CMS quality programs would implement USCDI+ Quality elements.
USCDI v5 (July 2024) introduced two critical data classes that directly address medication errors and care coordination gaps. The Observations class includes Sex Parameter for Clinical Use (SPCU)—providing sex-specific context for lab results where normal ranges vary significantly by biological sex—and Advance Directive Observation for capturing end-of-life care preferences. The Orders class standardizes five order types: Medication, Laboratory, Diagnostic Imaging, Clinical Test, and Procedure Orders. Early adopters report 35% reductions in duplicate testing and 28% improvements in medication reconciliation accuracy. Version 5 became available for voluntary adoption through SVAP on August 29, 2025.
USCDI v6 (July 2025) promoted six mature elements from specialized USCDI+ programs: Care Plan (comprehensive treatment coordination), Portable Medical Order (DNR/POLST directives that follow patients across settings), Unique Device Identifier (implanted device tracking for recalls and safety), Facility Address (standardized location data), Date of Onset (symptom timeline for diagnosis), and Family Health History (genetic risk factors). These elements proved their value in focused implementations before broader adoption. Organizations implementing v6 elements report 42% improvements in patient adherence to complex treatment plans and 89% better compliance with portable medical orders.
Implement now if you prioritize care coordination, patient safety, or competitive differentiation. While v3 is mandatory as of January 1, 2026, v5 and v6 offer measurable advantages: organizations document $125,000 annual savings per 100 beds from reduced duplicate testing, 28% better medication reconciliation, and 340% ROI within 18 months. Most major EHR vendors (Epic, Cerner Oracle Health, Meditech, athenahealth) already support v5 through SVAP—requiring configuration, not system replacement. Early adopters optimize workflows gradually and build expertise that becomes competitive advantage, while organizations waiting for mandates face rushed implementations with compressed timelines. If you're resource-constrained and focused solely on minimum compliance, ensure robust v3 implementation by January 2026 and plan v5 adoption within 12-18 months.











.png)

.png)

.png)